general contraindications to combined oral hormonal contraceptive
Last edited 03/2021 and last reviewed 03/2021
These include:
- Consent not given.
- Individuals under 16 years of age and assessed as not competent using Fraser Guidelines.
- Individuals 16 years of age and over and assessed as lacking capacity to consent.
- Known or suspected pregnancy.
- Known hypersensitivity to the active ingredient or to any constituent of the product - see Summary of Product Characteristics
- Less 21 days after childbirth (for deliveries over 24 weeks gestation)
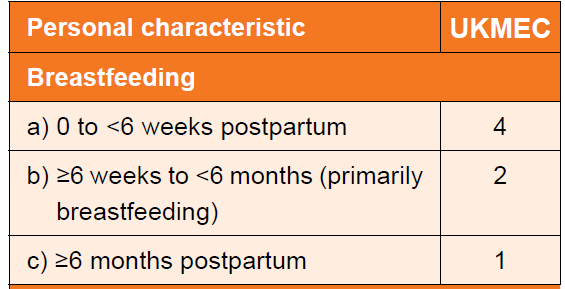
- Breastfeeding and less than six weeks postpartum.
- Not breastfeeding and 3-6 weeks post-partum with other risk factors for venous thromboembolism (VTE).
- Individuals aged 50 years and over.
Cardiovascular disease
- Individuals aged 35 years or more and smoking or stopped smoking less than one year ago
- Body Mass Index (BMI) equal to or greater than 35kg/m2
- Blood pressure greater than 140/90mmHg or controlled hypertension
- Multiple risk factors for cardiovascular disease (CVD) (such as smoking, diabetes, hypertension, obesity and dyslipidaemias)
- Current or past history of ischaemic heart disease, vascular disease, stroke or transient ischaemic attack
- Current or past history of venous thromboembolism
- Complicated valvular or congenital heart disease e.g. pulmonary hypertension, history of subacute bacterial endocarditis
- First degree relative with venous thromboembolism under 45 years of age
- Known thrombogenic mutations e.g. factor V Leiden, prothrombin mutation, protein S, protein C and antithrombin deficiencies
- Cardiomyopathy with impaired cardiac function
- Atrial fibrillation
- Significant or prolonged immobility.
- Imminent planned major surgery (COC should be stopped at least 4 weeks prior to planned major surgery or expected period of limited mobility).
Neurological Conditions
- Current or past history of migraine with neurological symptoms including aura at any age
- Migraine without aura, first attack when on method of contraception containing an estrogen
Cancers
- Past or current history of breast cancer
- Undiagnosed breast mass (for initiation of method only)
- Carrier of known gene mutations associated with breast cancer e.g. BRCA1or 2
- Malignant liver tumour (hepatocellular carcinoma)
Gastro-intestinal Conditions
- Viral hepatitis, acute or flare (for initiation only)
- Severe decompensated cirrhosis
- Gall bladder disease, symptomatic, medically treated
- Gall bladder disease, currently symptomatic
- Any bariatric or other surgery resulting in malabsorption.
- Cholestasis (related to past combined hormonal contraceptive use)
- Benign liver tumour (hepatocellular adenoma)
Other conditions
- Diabetes with end organ disease (retinopathy, nephropathy, neuropathy)
- Positive anti-phospholipid antibodies (with or without systemic lupus erythematosus)
- Organ transplant, with complications
- Individuals using enzyme-inducing drugs/herbal products or within 4 weeks of stopping them.
- Known severe renal impairment or acute renal failure
- Acute porphyria
- breastfeeding - UKMEC score varies with respect to timing of use of combined hormonal contraception (2)
Reference:
- Patient Group Direction (PGD) (NHS Specialist Pharmacy Service). Supply of a combined oral hormonal contraceptive (COC) . (Accessed 17th March 2021).
- FSRH Guideline (January 2019; amended November 2020). Combined Hormonal Contraception
COC and cardiovascular disease (CVD) risk
arterial thrombosis in the lower limb
Dubin-Johnson syndrome and the combined contraceptive (COC) pill