CANVAS Program - Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes
Last edited 10/2018 and last reviewed 08/2023
CANVAS Program - Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes
CANVAS Program integrated data from two trials involving a total of 10,142
participants with type 2 diabetes and high cardiovascular risk
- participants in each trial were randomly assigned to receive canagliflozin or placebo and were followed for a mean of 188.2 weeks
- primary outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke
- the CANVAS Program was a prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials (1,2)
Results:
- mean age of the participants was 63.3 years, 35.8% were women
- mean duration of diabetes was 13.5 years, and 65.6% had a history of cardiovascular disease
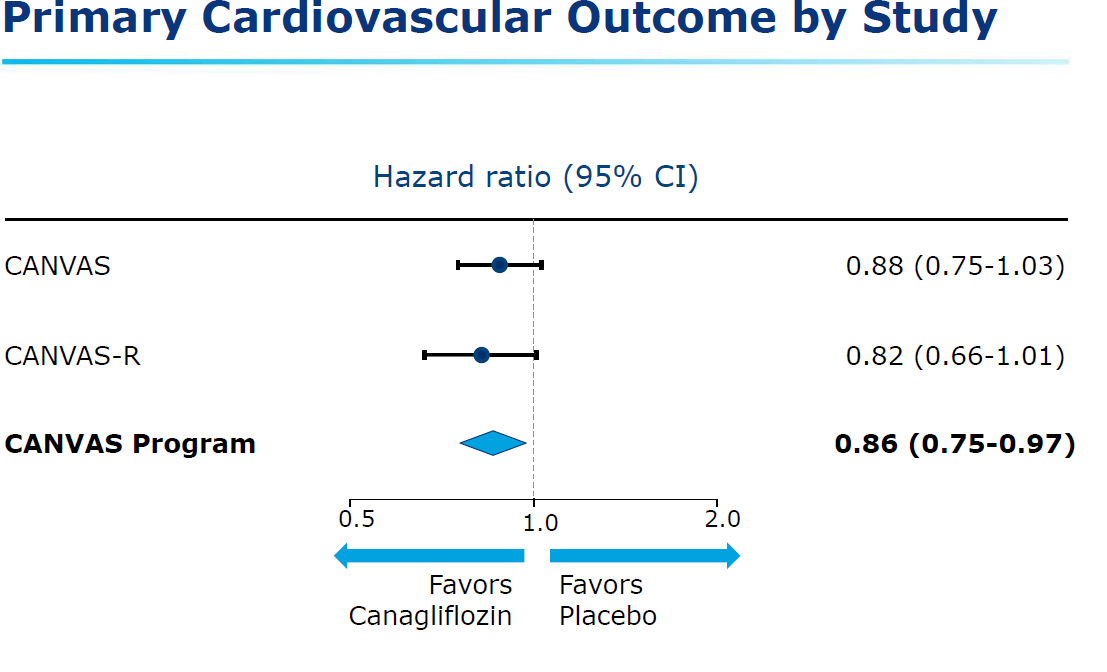
- rate of the primary outcome was lower with canagliflozin than with placebo (occurring in 26.9 vs. 31.5 participants per 1000 patient-years; hazard ratio, 0.86; 95% confidence interval [CI], 0.75 to 0.97; P<0.001 for noninferiority; P=0.02 for superiority)
- the primary cardiovascular outcome for the CANVAS and CANVAS-R studies individually
did not reach significance. However for the CANVAS Program the primary cardiovascular
endpoint did reach significance
- although on the basis of the prespecified hypothesis testing sequence the renal outcomes are not viewed as statistically significant, the results showed a possible benefit of canagliflozin with respect to the progression of albuminuria (hazard ratio, 0.73; 95% CI, 0.67 to 0.79) and the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate, the need for renal-replacement therapy, or death from renal causes (hazard ratio, 0.60; 95% CI, 0.47 to 0.77)
- adverse reactions were consistent with the previously reported risks associated with canagliflozin except for an increased risk of amputation (6.3 vs. 3.4 participants per 1000 patient-years; hazard ratio, 1.97; 95% CI, 1.41 to 2.75); amputations were primarily at the level of the toe or metatarsal
The study authors concluded:
- "...in two trials involving patients with type 2 diabetes and an elevated risk of cardiovascular disease, patients treated with canagliflozin had a lower risk of cardiovascular events than those who received placebo but a greater risk of amputation, primarily at the level of the toe or metatarsal..."
Key findings:
- CANVAS Program met its primary objective of demonstrating the cardiovascular safety and efficacy of canagliflozin
- canagliflozin use was associated with an increased risk of amputation which should be taken into consideration when prescribing this agent
- data suggest a favorable benefit/risk profile with canagliflozin treatment in many patients with type 2 diabetes and high cardiovascular risk
Reference:
- Neal B et al. Optimizing the analysis strategy for the CANVAS Program: A prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials.Diabetes Obes Metab. 2017 Jul;19(7):926-935
- Neal B et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes.N Engl J Med. 2017 Aug 17;377(7):644-657
- Neal B et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS) - a randomized placebo-controlled trial. Am Heart J 2013;166:217-223.e11
- Neal B et al. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes Metab 2017;19:387-393
EMPA - REG trial - empagliflozin in type 2 diabetes patients with high cardiovascular risk (EMPAREG)
DECLARE - TIMI 58 - dapagliflozin and cardiovascular outcomes in type 2 diabetes
CREDENCE - canagliflozin and renal outcomes in type 2 diabetes (diabetic) and nephropathy