Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA-HF)
Last edited 09/2019 and last reviewed 06/2023
Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA-HF)
DAPA-HF trial enrolled 4744 HFrEF patients (symptomatic HF, EF <=40%, NT-proBNP >= 600 pg/mL) and those with eGFR <30 mL/min/1.73m2, symptomatic hypotension, SBP <95 mmHg or type 1 diabetes were excluded.
Primary endpoint was worsening HF event (unplanned HF hospitalization or an urgent HF visit requiring IV therapy) or CV death.
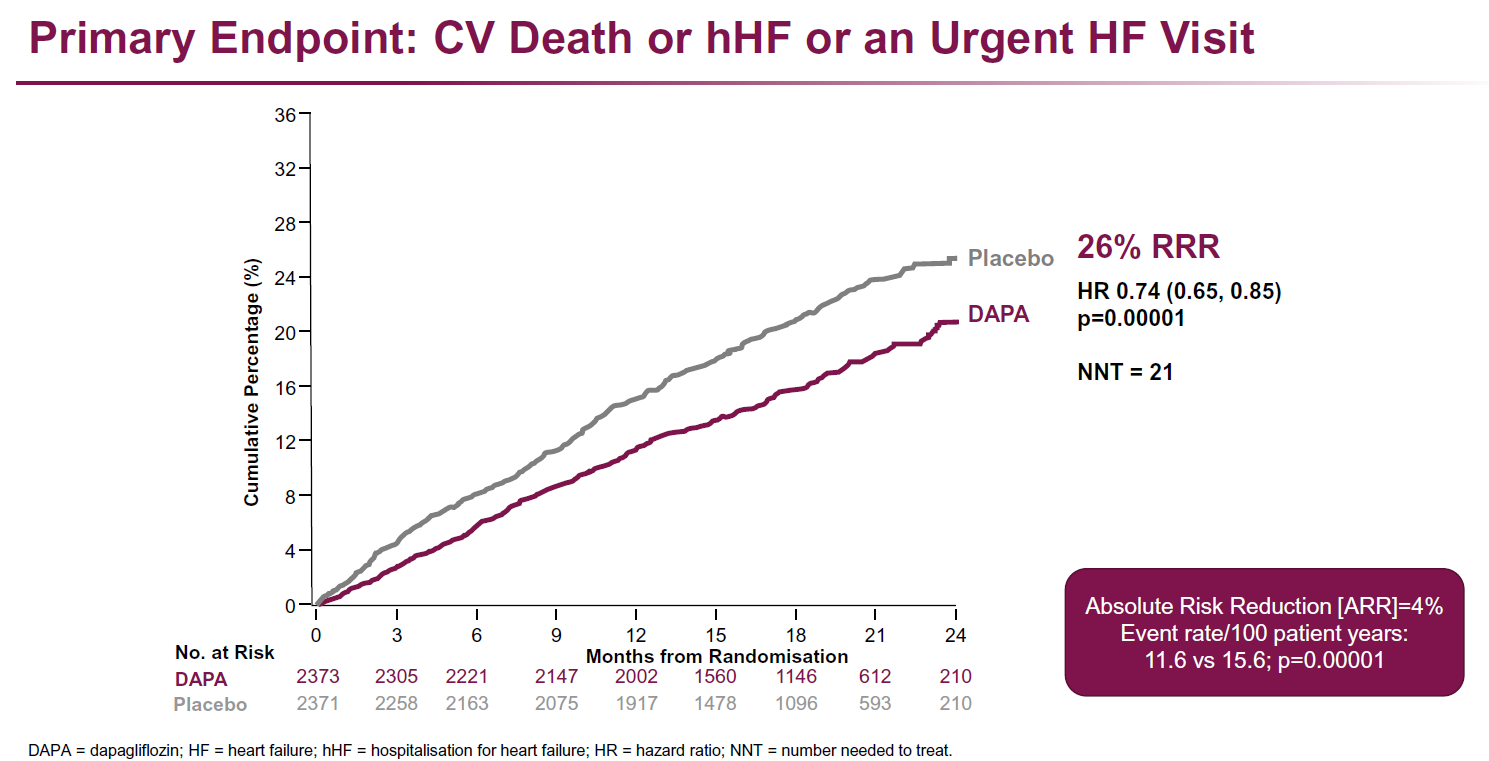
Main results
- Treatment with dapagliflozin resulted in a reduction of the primary endpoint of CV death and worsening HF event compared to placebo (HR: 0.74, 95%CI: 0.65-0.85, P=0.00001, NNT=21)
- All-cause death was reduced in the dapagliflozin group compared to the placebo group (HR: 0.83, 95%CI: 0.71-0.97, P=0.022). In the dapagliflozin group, percentage of patients with >= 5 point improvement in the Kansas City Cardiomyopathy Questionnaire (KCCQ) was increased and percentage of patients with >= 5 point deterioration was lowered compared to the placebo group.
- Individual endpoints (HF hospitalization/urgent HF visit, HF hospitalization, CV death, CV death/HF hospitalization, death from any cause) were all reduced in those who were on dapagliflozin compared to those who were on placebo. Dapagliflozin was well tolerated.
Regarding side effects, 178 patients (7.5%) in the dapagliflozin group had an adverse event related to volume depletion compared to 162 (6.8%) in the placebo group, with no significant difference between groups. Adverse events related to renal dysfunction occurred in 153 patients (6.5%) in the dapagliflozin group versus 170 patients (7.2%) in the placebo group, with no significant difference between groups. Major hypoglycaemia and lower limb amputation and fracture were infrequent and occurred at similar rates in the two treatment groups.
Dapagliflozin reduced the composite outcome of CV death and worsening HF event in HFrEF patients with and without diabetes in comparison to placebo. In addition, CV death was reduced and symptoms improved with dapagliflozin therapy compared to placebo

Commentary (2):
- "DAPA-HF could be "tectonic" in how it affects care of patients with
heart failure - with or without diabetes.
- DAPA-HF involved the use of dapagliflozin 10mg or placebo to patients with symptomatic heart failure (having an ejection fraction of less than 40%). This was in addition to optimum care with drugs prescribed from primary care such as beta blockers, diuretics and ACE inhibitors; as well as other therapies such as sacubutril.
- The headline from the study was - over a median of just over 18 months, there was a relative risk reduction of the primary endpoint of cardiovascular death or worsening heart failure of 22% in the dapa treated patient group - a NNT of 21.
- There was also no difference in incidence of significant hypoglycaemia in comparison to placebo or dapa treated individuals.
- This study says that in addition to optimum therapy, adding dapagliflozin will reduce the risk of cardiovascular death or worsening of heart failure by 1/5 - and this effect shown in a relatively short study. Obviously, this is the first of the studies of SGLT2s in heart failure (and others are in the trial pipeline) but I am certain GPs will shortly (if not already) be seeing dapagliflozin 10mg being prescribed to patients with heart failure by cardiology teams."
Reference:
- McMurray JVV et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019 Sep 19. doi: 10.1056/NEJMoa1911303
- Commentary - Dr Jim McMorran (Editor in Chief, GPnotebook) - September 22nd 2019.
DAPA HF - analysis of risk of new onset type 2 diabetes in patients with HFrEF
dapagliflozin - NICE guidance for treating chronic heart failure with reduced ejection fraction
DAPA HF - analysis reveals evidence of benefit of dapagliflozin apparent 28 days post-initiation
DELIVER study - dapagliflozin in heart failure with mildly reduced or preserved ejection fraction
DELIVER study - dapagliflozin in heart failure with mildly reduced or preserved ejection fraction