UK Medical Eligibility for Contraceptive Use (UKMEC , contraception) and (BMI (>=30), obesity) advice
Last edited 03/2020 and last reviewed 05/2021
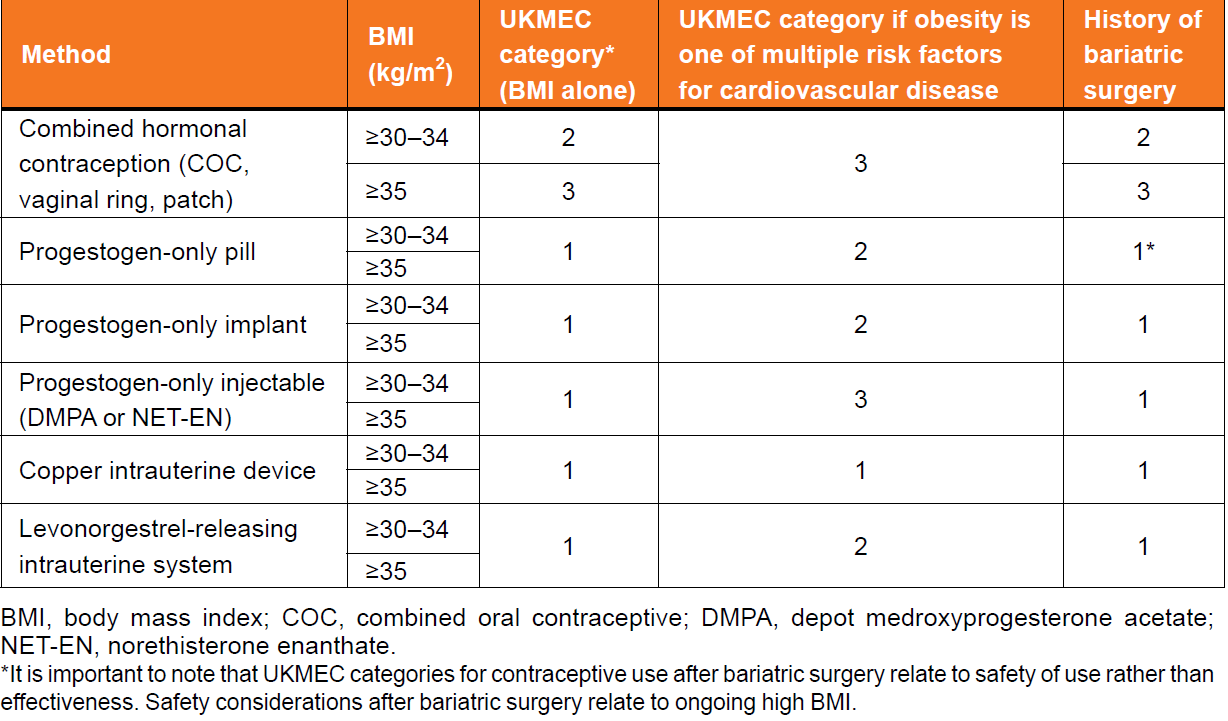
UKMEC guidance and obesity
For women with obesity (BMI categories of >=30-34 kg/m2 and >=35 kg/m2) without coexistent medical conditions
- the UKMEC categorises all progestogen-only contraceptives and intrauterine contraception as UKMEC 1, which means that there are no restrictions on the use of these methods.
For women with obesity (BMI categories of >=30-34 kg/m2 and >=35 kg/m2) of all ages, all estrogen containing contraception (i.e. combined hormonal contraception (CHC), including combined oral contraception (COC) containing both ethinylestradiol (EE) and estradiol, patch and ring)
- categorised as UKMEC 2 or 3, depending on BMI. These categorisations are primarily because of increased risk of VTE.
For women with raised BMI with other risk factors for CVD in addition to obesity (e.g. smoking, diabetes, hypertension and dyslipidaemias)
- the copper intrauterine device (Cu-IUD) remains UKMEC 1
- the levonorgestrel-releasing intrauterine system (LNG-IUS), contraceptive implants and the progestogen-only pill (POP) are UKMEC 2
- progestogen-only injectables (depot medroxyprogesterone acetate (DMPA) and norethisterone enanthate (NET-EN)) and CHC are classed as UKMEC 3.
It is important to note that UKMEC categories for contraceptive use after bariatric surgery relate to safety of use rather than effectiveness. Safety considerations after bariatric surgery relate to ongoing high BMI - see linked item
UK Medical Eligibility Criteria for Contraceptive Use (UKMEC) categories based on body mass index:
Reference:
UKMEC (UK Medical Eligibility for Contraceptive Use) criteria
intrauterine contraception (IUC) and obesity
progestogen only implant (contraceptive) (contraception) and obesity
progestogen only injectable contraceptive (contraception) and obesity
progestogen only pill (POP) and obesity