combined contraceptive pill and migraine
Last edited 06/2020 and last reviewed 11/2020
Patients should be warned to report increase in headache frequency or onset of focal symptoms - the combined pill should be discontinued and the patient urgently referred if focal neurological symptoms not typical of aura persist for 1 hour or more.
The combined pill is contra-indicated in:
- patients who suffer regular severe migraines lasting more than 72 hours despite treatment
- patients who suffer migraines with typical focal aura
- in patients with migraines without aura if there more than one additional risk factor for arterial disease (1)
- if migraine is treated with ergot derivatives
The combined pill can be prescribed with caution in patients who:
- have migraines without typical focal aura
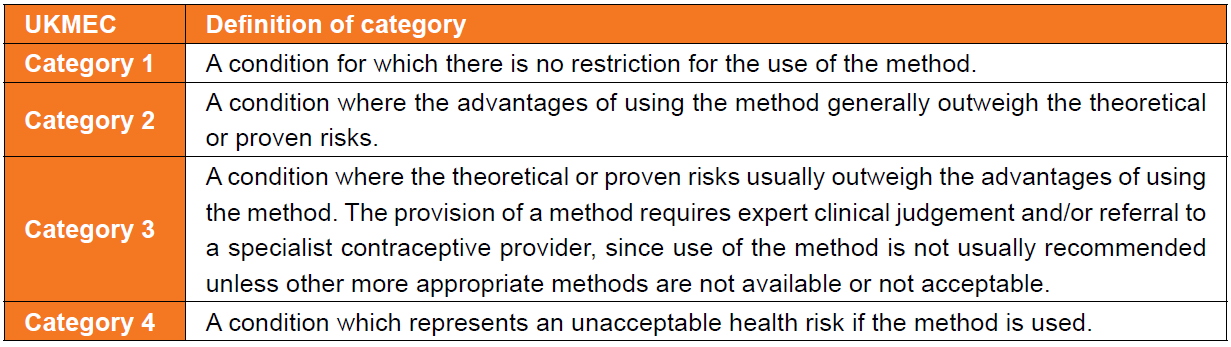
UKMEC Criteria state:
In consideration of UKMEC criteria and combined hormonal contraception (CHC):
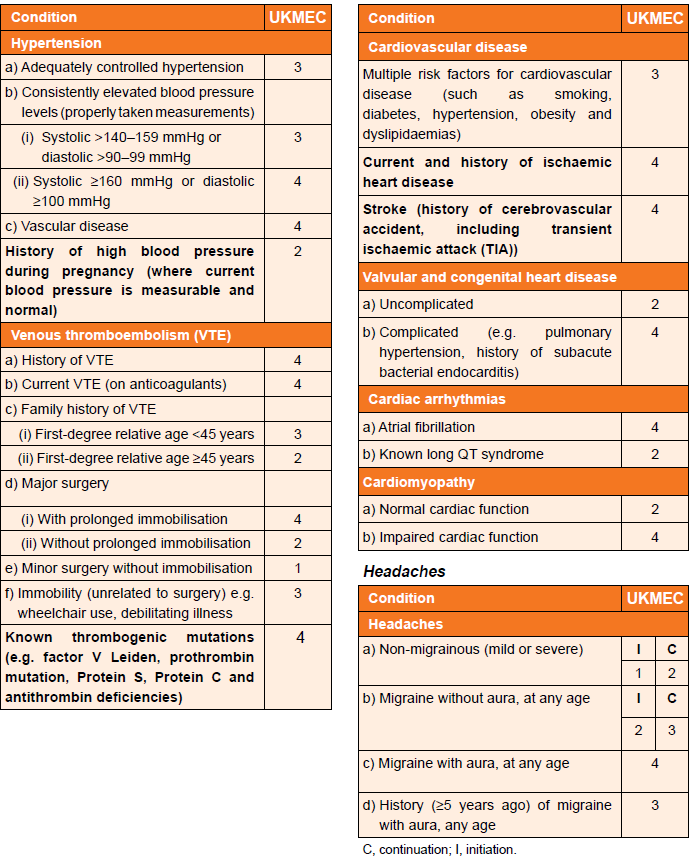
Cardiovascular factors (including migraine) and UKMEC categories (3):
Check the summary of product characteristics before prescribing any combined oral contraceptive pill.
Reference:
- (1) DTB (2000), 38 (1), 1-4.
- (2) BNF 7.3
- (3) FSRH Clinical Guideline: Combined Hormonal Contraception (January 2019, Amended July 2019)
choice of oral contraceptive in patient with migraine
oral contraceptive pill (risk factors for arterial disease)