non-inferiority and superiority trials
Last edited 12/2021 and last reviewed 12/2021
Non-Inferiority and Superiority Trials
The objective of non-inferiority trials is to compare a novel treatment to an active treatment with a view of demonstrating that it is not clinically worse with regards to a specified endpoint. It is assumed that the comparator treatment has been established to have a significant clinical effect (against placebo).
These trials are frequently used in situations where use of a superiority trial against a placebo control may be considered unethical.
Non-inferiority is most easily assessed using a confidence interval (CI) approach.
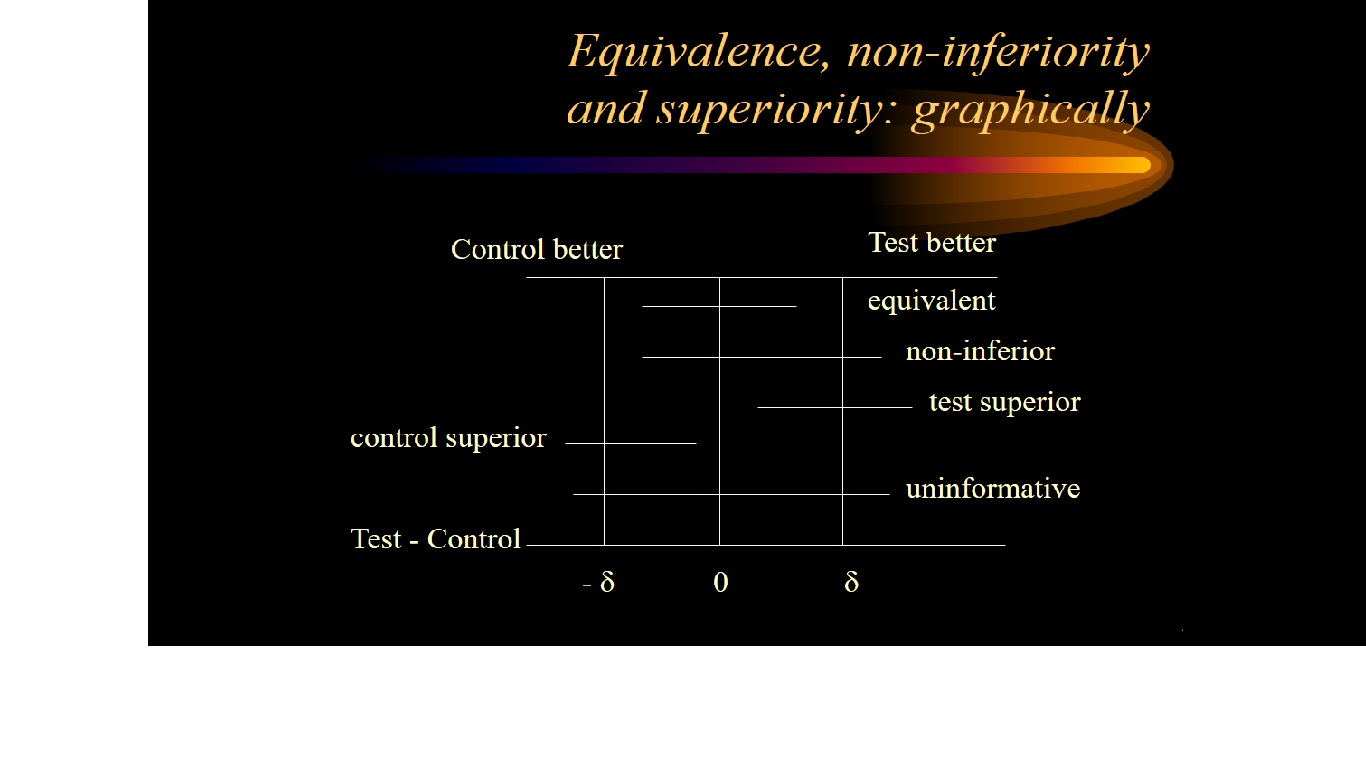
Firstly the trial investigators specify a non-inferiority margin, delta. This is the maximum difference that they are prepared to tolerate in a given direction if the new treatment is not to be considered (clinically) inferior.
If a 95% confidence interval for the difference between treatment means lies above or below this boundary value (in a favourable direction) then non-inferiority is deemed to have been established.
Logic of Non-Inferiority studies
- If a standard S is consistently superior to placebo, then
- to show that a test treatment T is superior to placebo
- it suffices to show that the test treatment is as good as (not inferior) to the standard
Setting the non-inferiority margin
- subjective - often contentious
- if too large:
- inferior treatments may be called non-inferior
- if too small: huge sample sizes are required
- usually a fraction of the historical difference between control and placebo
Interpreting a noninferiority trial as a superiority trial
- if the 95% confidence interval for the treatment effect not only lies entirely
above -delta but also above zero then there is evidence of superiority in
terms of statistical significance at the 5% level (P < 0.05)
- switching between superiority and noninferiority
- interpreting a noninferiority trial as a superiority trial is credible and without a need for a statistical penalty for multiple testing
- if the 95% CI for the treatment benefit excludes not only the noninferiority margin but also zero, it would be considered adequate evidence to prove superiority within the same trial
- however, the opposite approach is not valid
- if a superiority trial fails to reject the null hypothesis but the trial data appear to suggest treatment equivalence, one may also be tempted to infer noninferiority
- if there is a possibility for testing noninferiority alongside a superiority test, one should predefine both hypotheses with a justifiable margin for noninferiority in the protocol
- testing noninferiority based on an ad hoc determination of a noninferiority margin after a trial is complete would not be acceptable due to bias
- when both hypotheses are carefully planned within a protocol, both can be tested on the same population without a statistical penalty
- see diagram below:
- in this case it is acceptable to calculate the P value associated with a test of superiority and to evaluate whether this is sufficiently small to reject convincingly the hypothesis of no difference
- there is no multiplicity argument that affects this interpretation because,
in statistical terms, it corresponds to a simple closed test procedure
- usually this demonstration of a benefit is sufficient on its own, provided the safety profiles of the new agent and the comparator are similar. When there is an increase in adverse events, however, it is important to estimate the size of the effect to evaluate whether it is sufficient in clinical terms to outweigh the adverse effects
Reference: